-
Subscribe to Blog:
SEARCH THE BLOG
CATEGORIES
- Aerospace
- Asset Maintenance
- Automotive
- Blog
- Building Products
- Case Studies
- Chemical Processing
- Consulting
- Food & Beverage
- Forestry Products
- Hospitals & Healthcare
- Knowledge Transfer
- Lean Manufacturing
- Life Sciences
- Logistics
- Manufacturing
- Material Utilization
- Metals
- Mining
- News
- Office Politics
- Oil & Gas
- Plastics
- Private Equity
- Process Improvement
- Project Management
- Spend Management
- Supply Chain
- Uncategorized
- Utilities
- Whitepapers
BLOG ARCHIVES
- July 2025 (1)
- June 2025 (4)
- May 2025 (1)
- April 2025 (1)
- March 2025 (1)
- February 2025 (4)
- January 2025 (4)
- December 2024 (4)
- November 2024 (2)
- October 2024 (6)
- September 2024 (5)
- August 2024 (5)
- July 2024 (6)
- June 2024 (3)
- May 2024 (3)
- April 2024 (4)
- March 2024 (3)
- February 2024 (4)
- January 2024 (5)
- December 2023 (2)
- November 2023 (1)
- October 2023 (6)
- September 2023 (3)
- August 2023 (4)
- July 2023 (2)
- June 2023 (3)
- May 2023 (7)
- April 2023 (3)
- March 2023 (3)
- February 2023 (5)
- January 2023 (6)
- December 2022 (2)
- November 2022 (5)
- October 2022 (5)
- September 2022 (5)
- August 2022 (6)
- July 2022 (3)
- June 2022 (4)
- May 2022 (5)
- April 2022 (3)
- March 2022 (5)
- February 2022 (4)
- January 2022 (7)
- December 2021 (3)
- November 2021 (5)
- October 2021 (3)
- September 2021 (2)
- August 2021 (6)
- July 2021 (2)
- June 2021 (10)
- May 2021 (4)
- April 2021 (5)
- March 2021 (5)
- February 2021 (3)
- January 2021 (4)
- December 2020 (3)
- November 2020 (3)
- October 2020 (3)
- September 2020 (3)
- August 2020 (4)
- July 2020 (3)
- June 2020 (5)
- May 2020 (3)
- April 2020 (3)
- March 2020 (4)
- February 2020 (4)
- January 2020 (4)
- December 2019 (3)
- November 2019 (2)
- October 2019 (4)
- September 2019 (2)
- August 2019 (4)
- July 2019 (3)
- June 2019 (4)
- May 2019 (2)
- April 2019 (4)
- March 2019 (4)
- February 2019 (5)
- January 2019 (5)
- December 2018 (2)
- November 2018 (2)
- October 2018 (5)
- September 2018 (4)
- August 2018 (3)
- July 2018 (2)
- June 2018 (4)
- May 2018 (3)
- April 2018 (3)
- March 2018 (2)
- February 2018 (2)
- January 2018 (1)
- December 2017 (1)
- November 2017 (2)
- October 2017 (2)
- September 2017 (1)
- August 2017 (2)
- July 2017 (2)
- June 2017 (1)
- April 2017 (3)
- March 2017 (3)
- February 2017 (2)
- January 2017 (2)
- December 2016 (2)
- November 2016 (4)
- October 2016 (4)
- September 2016 (3)
- August 2016 (6)
- July 2016 (4)
- June 2016 (4)
- May 2016 (1)
- April 2016 (3)
- March 2016 (4)
- February 2016 (2)
- January 2016 (4)
- December 2015 (3)
- November 2015 (3)
- October 2015 (1)
- September 2015 (1)
- August 2015 (4)
- July 2015 (6)
- June 2015 (4)
- May 2015 (7)
- April 2015 (6)
- March 2015 (6)
- February 2015 (4)
- January 2015 (3)
CONNECT WITH US
Tag Archives: process control
Achieving efficiency in manufacturing requires meticulous attention to pre-production processes, especially when managing temperature-sensitive operations. Pre-manufacturing thermal management is essential for maintaining product quality, ensuring equipment longevity, and improving overall operational efficiency.
The Role of Thermal Management in Manufacturing
Thermal management involves regulating temperature levels within machinery, materials, and environments to create ideal conditions for production. Excessive heat or improper cooling can compromise machinery performance and lead to defects in temperature-sensitive products. A robust thermal management strategy minimizes these risks, ensuring consistent outcomes and reducing downtime caused by equipment failure.
Industries such as electronics, pharmaceuticals, and aerospace often handle materials that demand precise thermal control. For instance, electronic components require steady temperatures during assembly to avoid warping or damage. Without adequate thermal management, manufacturers risk product recalls and damaged reputations.
Pre-Manufacturing Strategies for Temperature Control
Implementing a pre-manufacturing thermal management plan involves understanding your facility’s specific needs and employing the right tools to monitor and maintain conditions. Thermal analysis equipment is a key investment for businesses aiming to achieve optimal production outcomes. These tools provide detailed insights into how heat is distributed and managed throughout the production process, helping identify areas of inefficiency or potential failure.
Effective thermal management strategies also include proper ventilation systems, insulation, and advanced cooling technologies. Additionally, scheduling routine maintenance ensures that thermal management tools operate correctly, preventing unexpected disruptions during production.
The Long-Term Benefits of Optimal Thermal Management
Businesses that prioritize pre-manufacturing thermal control enjoy several advantages, including reduced operational costs, improved product quality, and extended equipment lifespans. By addressing thermal issues early, companies can avoid costly repairs, minimize energy consumption, and enhance workplace safety.
Furthermore, implementing thermal management measures aligns with sustainability goals, as efficient temperature regulation often reduces waste and energy usage, positively impacting the environment.
Pre-manufacturing thermal management is more than just a technical requirement—it’s a cornerstone of efficient and sustainable production. Investing in tools and prioritizing proactive strategies ensures businesses can meet high-quality standards while staying competitive in a fast-paced market.
Check out the accompanying resource below to learn more.
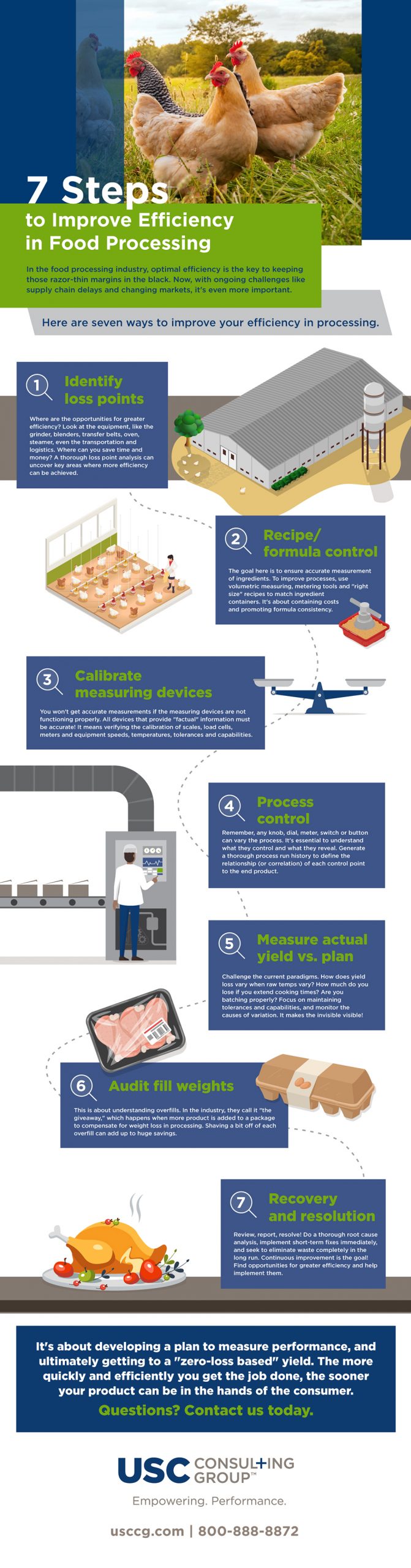
In the food processing industry, optimal efficiency is the key to keeping those razor-thin margins in the black. Now, with ongoing challenges like supply chain delays and changing markets, it’s even more important.
Efficient processes lead to reduced operating costs, improved yield and throughput, and more control over your management systems.
The following graphic provides seven ways to improve your efficiency in food processing:
1. Identify loss points
Where are the opportunities for greater efficiency? Look at the equipment, like the grinder, blenders, transfer belts, oven, steamer, even the transportation and logistics. Where can you save time and money? A thorough loss point analysis can uncover key areas where more efficiency can be achieved.
2. Recipe/formula control
The goal here is to ensure accurate measurement of ingredients. To improve processes, use volumetric measuring, metering tools and “right size” recipes to match ingredient containers. It’s about containing costs and promoting formula consistency.
3. Calibrate measuring devices
You won’t get accurate measurements if the measuring devices are not functioning properly. All devices that provide “factual” information must be accurate! It means verifying the calibration of scales, load cells, meters and equipment speeds, temperatures, tolerances and capabilities.
4. Process control
Remember, any knob, dial, meter, switch or button can vary the process. It’s essential to understand what they control and what they reveal. Generate a thorough process run history to define the relationship (or correlation) of each control point to the end product.
5. Measure actual yield vs. plan
Challenge the current paradigms. How does yield loss vary when raw temps vary? How much do you lose if you extend cooking times? Are you batching properly? Focus on maintaining tolerances and capabilities, and monitor the causes of variation. It makes the invisible visible!
6. Audit fill weights
This is about understanding overfills. In the industry, they call it “the giveaway,” which happens when more product is added to a package to compensate for weight loss in processing. Shaving a bit off of each overfill can add up to huge savings.
7. Recovery and resolution
Review, report, resolve! Do a thorough root cause analysis, implement short-term fixes immediately, and seek to eliminate waste completely in the long run. Continuous improvement is the goal! Find opportunities for greater efficiency and help implement them.
It’s about developing a plan to measure performance, and ultimately getting to a “zero-loss based” yield. The more quickly and efficiently you get the job done, the sooner your product can be in the hands of the consumer. Questions? Contact us today.
For more information about how Food & Beverage consultants can significantly reduce your operating costs and improve productivity, read this eBook:
Pharmaceutical manufacturers can magnify operational efficiency by taking a closer look at on-site quality control processes.
Pharmaceutical manufacturing is all about innovation. The businesses within the industry work diligently to produce effective and accessible medications, as well as medical equipment for patients struggling with illnesses or discomfort. In this way, pharmaceuticals stand apart from other fields of manufacturing. Pharmaceutical can be ingested by customers or, as is the case with hypodermic needles or dialysis machines, connect invasively. As such, raw materials and finished products must be meticulously tested for quality to ensure patients receive healthy treatment doses, and drug manufacturers don’t accidentally introduce microbial pathogens into a patient’s already compromised immune system.
In finding new ways to increase operational efficiency in quality control testing labs for pharmaceutical manufacturers, the industry can enjoy advantages other industries do not – namely, the enhanced ability to save lives. However, these changes must be integrated carefully. Quality control professionals cannot make concessions on rigorously regulated testing assays. That said, finding methods for augmenting how the quality control process is performed – as opposed to the process itself – can yield powerful results in how pharmaceutical manufacturers retain valuable resources and expedite lead times, while still adhering to current good manufacturing practices (cGMP).
“Top performers in pharma had higher success rates with their medications.”
Understanding the value of communication
Before discussing a couple of efficiency measures the pharmaceutical industry could adopt to optimize performance in its clean rooms and testing labs, it is important to first touch on the true value of doing so. Drug companies take an incredible amount of time researching, testing, and producing their wares. A 2014 industry report from the International Federation of Pharmaceutical Manufacturers and Associations stated the time span between the start and end of a drug or vaccines research and development stages could be as much as 10 to 15 years per product. Even though these businesses want to develop as many treatment options as possible to generate a profit and help a widespread audience, they must adhere to all quality control standards, which can legitimately impact the rate of medical innovation.
Pharmaceutical manufacturers with similar time-efficiency issues could benefit from heightened attention to how the communicate inter-departmentally, or rather, how often these department communicate. Customer demand informs manufacturers on how they should produce and what they should be producing to meet the needs of consumers. As a business within the pharmaceutical industry accelerates its production cycles or scales its market share, data inherently becomes more granular and, indirectly, more volatile if left unchecked. While drug companies toy with the idea of addressing deficiencies in their quality control labs, they should first structure how these departments communicate, especially the frequency with which QC professionals sit down for meetings with other department heads. Weekly conversations, even informal ones, can keep everyone – including the laboratory – abreast of major production crunches or fluctuations in volume that could impact testing. For instance, if an executive announces the future construction of a new clean room in which manufacturing workers can develop treatments, this ultimately means the quality control lab will have an extra area to monitor regularly.
 How can manual testing methods receive a performance boost with help from rapid microbiology?
How can manual testing methods receive a performance boost with help from rapid microbiology?
Reducing costs through rapid microbiological methods
Cost reduction in pharmaceutical manufacturing does more than save businesses money – the action can have untold effects on U.S. healthcare. A study published in the Journal of Therapeutic Innovation and Regulatory Science revealed if drug companies decreased manufacturing costs by 30 percent, they could yield anywhere between $1 trillion and $12.3 trillion in social benefits to patients and medical science alike.
“Rapid microbiological equipment automates low-value laboratory labor.”
quality control testing is both a cost- and resource-intensive operation, principally so long as manual, growth-based assay remain the norm. However, the burgeoning field of rapid microbiology has been changing the ways quality control professionals function in a laboratory setting. As the name suggests, rapid microbiology seeks to increase the speed and efficacy with which quality control lab technicians perform their daily duties without altering time-tested compendial culture counting techniques. Additionally, rapid microbiological equipment automates certain low-value laboratory labor to free up technicians and centralize these processes into a single location.
To put rapid microbiological methods (RMM) into context, let’s focus on one specific laboratory activity: moving samples between incubators. Traditionally, lab workers would need to manually move the plates one by one at a predetermined time. Automated equipment could be programmed to execute this task with computer precision without much more than informal oversight.
According to a case study performed by Microbiology Consultants, LLC, a company that expended more than $5.2 million in conventional testing costs saw a nearly 90 percent reduction in operating costs the first year it switched to RMM. It is worth noting a significant portion of this reduction came from negating consumables and disposal costs entirely from the quality control process, thanks to innovative new approaches to sample preparation. Beyond the potential for return on investment (ROI), rapid microbial testing technology and strategies could insure against wasting valuable human capital, protect against contamination, and ultimately, shorten time to release while reinforcing quality control-related cGMP.
Integrating cost and resource efficiency into a pharmaceutical provider’s quality control model will require an unquestionable balance between eliminating waste and upholding best practices for the sake of its patients. Businesses who develop and integrate strong strategies for how to walk that line will benefit greatly.